Uranium series and secular equilibrium
In principle, the gamma-ray spectrum that one measures coming from 238-Uranium, is built up by virtually hundreds of gammaray energies emitted bij the nuclides in the 238-U decay series (more on this in A primer on gamma radiation). In a closed (sealed) environment, the nuclides in the series are said to be in equilibrium with the mother nucleus, 238U. This means that for any isotope in the series, the production rate equals the decay rate, and consequently a measurement of the activity (in terms of their rate) of one of them yields the activity of the mother. This is what is called "secular equilibrium".
However, if one of the nuclides in the series is taken away from the chain, the equilibrium situation is broken, and a measurement of the activity of this nuclide cannot any longer be used to estimate the activity of the mother. In this case, we are facing an out-of-equilibrium situation.
For the 238U-series this is a very common situation as one of the daughters in the series is 222Rn, aka Radon, a radioactive gas with a half-life of about 3.82 days. Uranium-containing minerals or soils are constantly producing this gas which - depending on the situation - can escape from the minerals and break the decay chain.
DIsequilibrium and gamma-ray spectra
One of the questions people often ask is wether gamma-ray spectra measured in the field with a scintillator detector can be used to determine wether a certain source is in equilibrium or not. The truth is that such determination will be pretty difficult. This can be understood by inspecting the table below listing a number of the gamma-ray energies emitted by the nuclides in the 238U-series. As one can see, the large majority (>99%) of the gamma-rays are coming from the decay of 214-Bismuth - a radon daughter. In other words, almost the full spectrum stems from a nuclide that is produced by Radon. There are a few lines that come from a nuclide "above" Radon, 234Pa. This nuclides emits a line at 1001 keV that could in principle be used to get an idea of the 238U activity, and the ratio of this peak w.r.t. the rest of the spectrum may reveal the breaking of the equilibrium conditions. However, one needs a high-resolution detector system to do this.
Sometimes there are other ways to determine wether a gammaray spectrum comes from 238U bound in the rock matrix, or from gaseous or dissolved radon. But that is the subject of another (pending) posting on this wiki. For now i'd like to refer to one of our whitepapers on the subject.
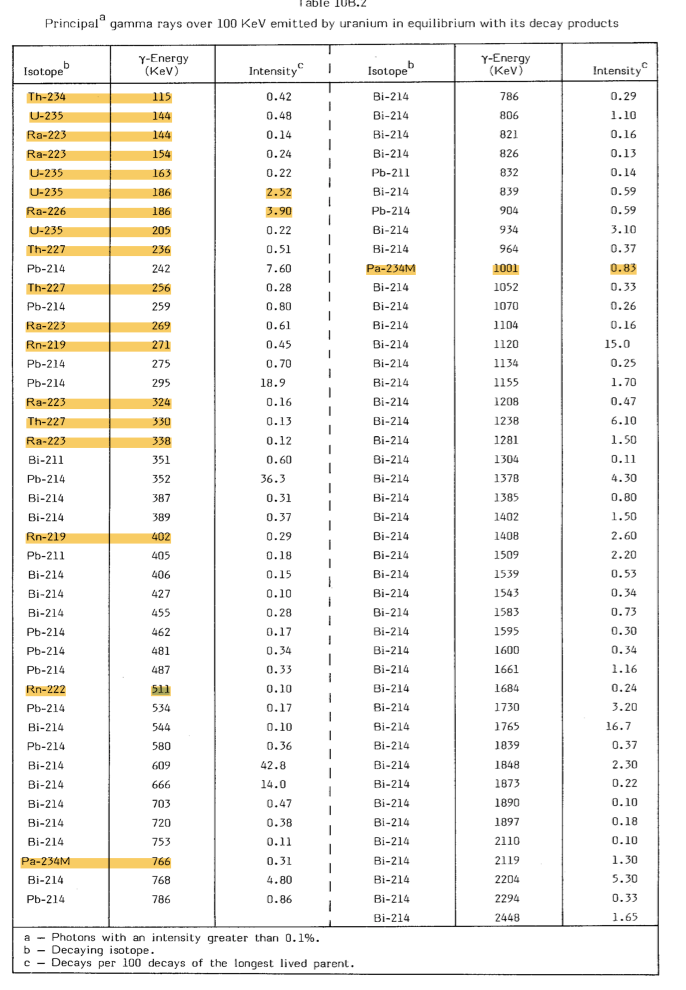
(Table taken from Grasty RL (1979) Gamma Ray Spectrometric Methods in Uranium Exploration - theory and Operational Procedures; in Geophysics and Geochemistry in the Search for Metallic Ores. Geol Sur Canada, Econ Geol Rep 31: 147-161.)
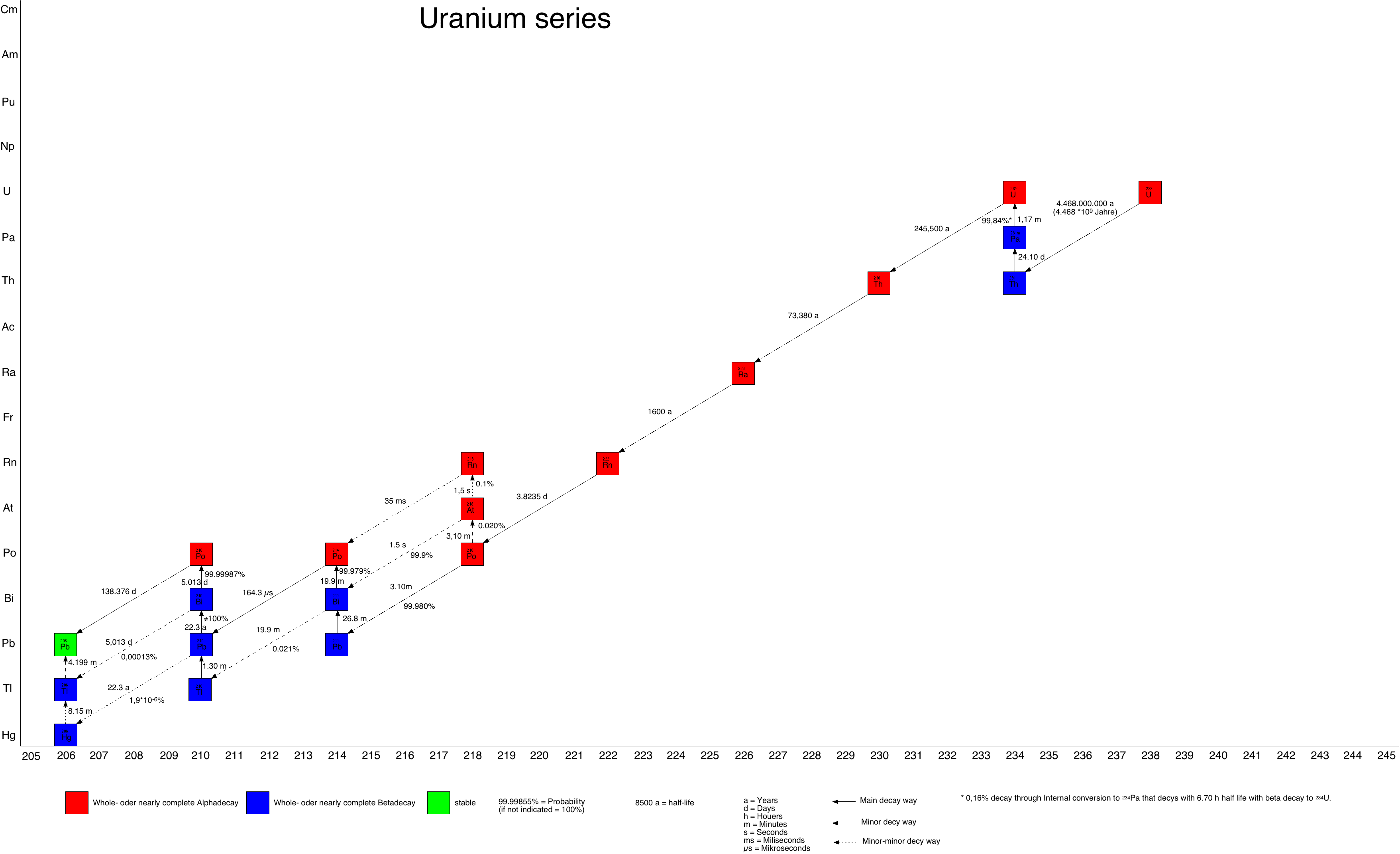
(Picture taken from https://en.wikipedia.org/wiki/Decay_chain)
