Natural radioactivity
In nature, most elements are stable and only a few naturally occurring radionuclides with a long half-life time are present. For our purpose the relevant radionuclides are: 40K and members of the decay series 232Th and 238U (see figure below). The half-life times of 238U and 232Th are longer than, or comparable to the age of the Earth, indicating that both nuclei are still present in the Earth’s crust.
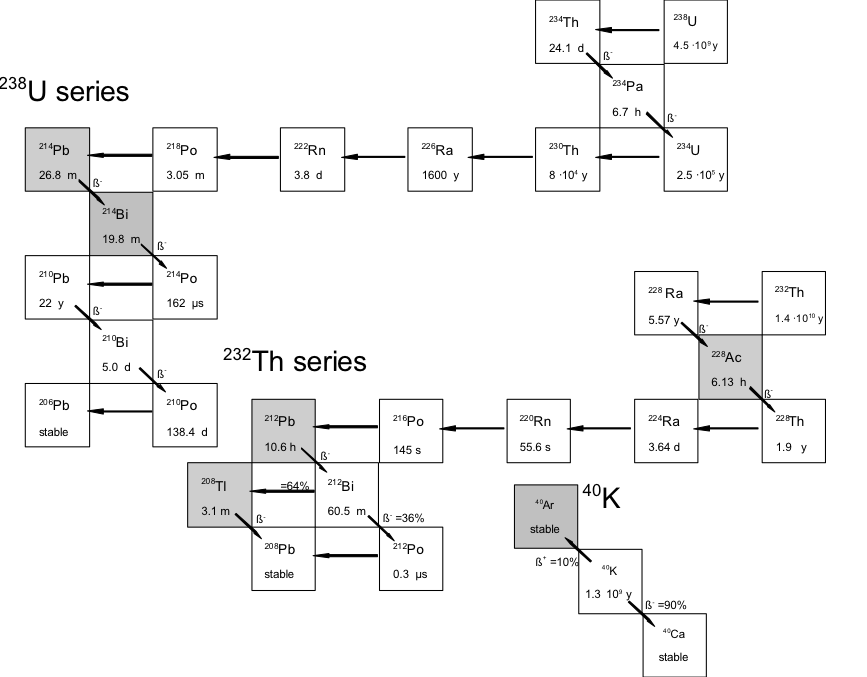
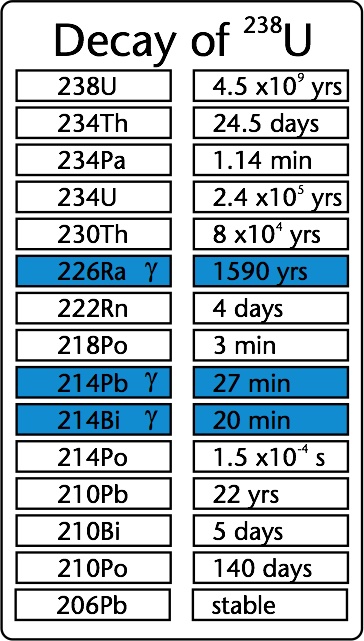
The decay chains of the three naturally occurring radionuclides are presented in the figure above. The emission of β-particles is denoted by diagonal lines, emission of 𝛼-particles occurs along the horizontal lines. The most relevant 𝛾-ray emitters are denoted by the grey boxes. For each nuclide, the half-life time is given. As can be seen, the half-lifes, and therefore the concentrations of the members of the decay series vary by a factor of 1015. Most of the members can therefore only be measured by radioactive decay since the concentrations are too low for chemical analyses .
Not all nuclei present in the decay chain emit 𝛾-rays. For the 238U series, the most important 𝛾-ray emitters are 214Pb and 214Bi and for the 232Th series, 228Ac, 212Pb and 208Ti. This means that concentrations of 238U and 232Th can be measured indirectly by the concentration of 𝛾-ray emitters from the decay chain provided that secular equilibrium is present. The breaking of secular equilibrium is mainly a concern in the 238U series. The decay series of 238U contains a radium isotope, soluble in water (226Ra) with a half-life time of 1600y. The next decay product is Radon (222Rn), which is a noble gas and has the possibility to escape the medium. If the system in which the measurements take place is not a closed system, which means that nuclei disappear not only due to radioactive decay, and radium and or radon can escape. Measuring g-rays from 214Pb and 214Bi will therefore underestimate the 238U concentration. To determine this effect of dis-equilibrium in sediment, concentrations of 238U in a zircon sample were measured with X-ray fluorescence, whilst the concentration of 214Bi was measured by g-ray spectroscopy. The results were compared and within the uncertainties, there were no indications that the systems were not closed (de Meijer et al., 1997), although it is known that 20-30% of radon formed in dry quartz sand does escape (van der Spoel, 1998). In water saturated conditions, radon remains close to its locations of formation and secular equilibrium is assumed.
